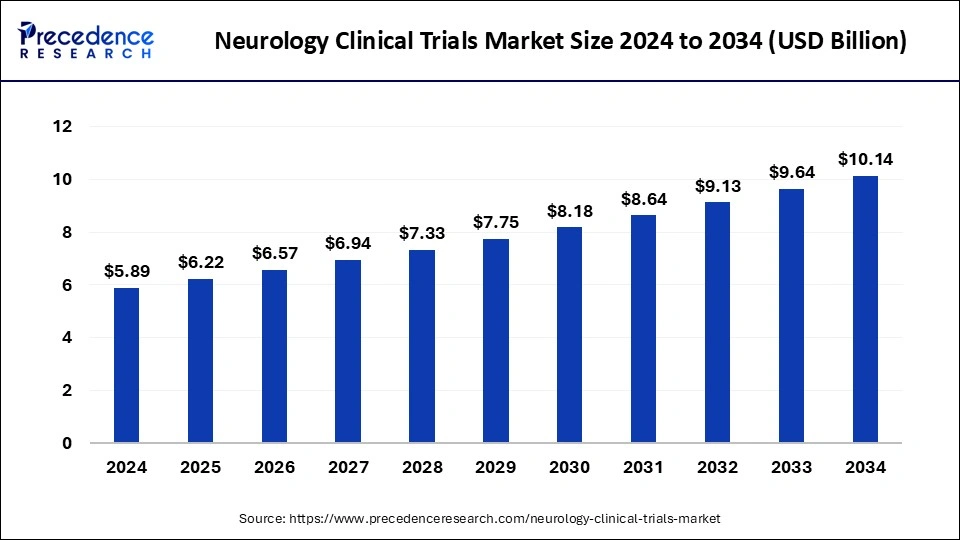
The global neurology clinical trials market size accounted for USD 5.58 billion in 2023 and is predicted to grow around USD 9.64 billion by 2033, expanding at a CAGR of 5.62% from 2024 to 2033.
Neurology Clinical Trials Market Key Points
- North America dominated the neurology clinical trials market with the largest revenue share of 48% in 2023.
- Asia Pacific is estimated to grow at a solid CAGR of 5.93% during the forecast period of 2024-2033.
- By phase, the phase II segment has generated more than 47% of revenue share in 2023.
- By phase, the phase III segment is projected to expand at a CAGR of 5.62% during the forecast period.
- By indication, the epilepsy segment has recorded more than 23% of revenue share in 2023.
- By indication, the huntington’s disease segment growing at a CAGR of 6.04% during the forecast period.
- By study design, the interventional segment dominated the market with the biggest revenue share of 96% in 2023.
- By study design, the observational segment is expected to grow at a CAGR of 5.83% during the forecast period.
The neurology clinical trials market focuses on the development and testing of new treatments for neurological disorders. These disorders encompass a wide range of conditions, including Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, epilepsy, and stroke. The market is driven by the increasing prevalence of these diseases, advancements in medical technology, and growing investments in research and development. Pharmaceutical companies, academic institutions, and contract research organizations (CROs) play significant roles in conducting these trials. The market is also influenced by regulatory frameworks and ethical considerations, which ensure the safety and efficacy of new treatments before they are made available to patients.
Get a Sample:https://www.precedenceresearch.com/sample/4657
Region Insights
Geographically, North America dominates the neurology clinical trials market, primarily due to the presence of major pharmaceutical companies, well-established healthcare infrastructure, and significant government funding for neurological research. The United States, in particular, leads the market with a high number of clinical trials and extensive research activities. Europe follows closely, with countries like Germany, the United Kingdom, and France being key contributors. The Asia-Pacific region is expected to witness substantial growth due to increasing healthcare investments, a large patient population, and rising awareness about neurological disorders. Countries like China, India, and Japan are emerging as important players in the market, with numerous clinical trials being conducted in these regions.
Trends
Several trends are shaping the neurology clinical trials market. One notable trend is the growing use of digital health technologies, such as wearable devices and remote monitoring, which enhance data collection and improve patient engagement. Another trend is the increasing adoption of personalized medicine approaches, which tailor treatments to individual genetic profiles and disease characteristics. Additionally, there is a rising focus on rare and orphan neurological diseases, with more clinical trials being dedicated to these conditions. The use of biomarkers and advanced imaging techniques to assess treatment efficacy is also gaining traction in the market.
Neurology Clinical Trials Market Scope
| Report Coverage | Details |
| Market Size by 2033 | USD 9.64 Billion |
| Market Size in 2023 | USD 5.58 Billion |
| Market Size in 2024 | USD 5.89 Billion |
| Market Growth Rate from 2024 to 2033 | CAGR of 5.62% |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | Phase, Study Design, Indication, Study Design, Phase, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Neurology Clinical Trials Market Dynamics
Drivers
The neurology clinical trials market is driven by several factors. The increasing prevalence of neurological disorders, due to aging populations and lifestyle changes, is a major driver. Advances in neuroimaging and molecular biology have improved the understanding of disease mechanisms, leading to the development of targeted therapies. The growing investment by pharmaceutical companies in neurology research and the availability of funding from government and private organizations also propel the market. Furthermore, the rising demand for innovative treatments and the need for improved therapeutic options for patients with neurological disorders contribute to the market’s growth.
Opportunities
The neurology clinical trials market presents numerous opportunities for growth. The development of novel therapeutics, such as gene therapies, stem cell treatments, and neuroprotective agents, offers promising avenues for clinical trials. The increasing focus on early diagnosis and intervention in neurological diseases creates opportunities for trials aimed at preventive treatments. Collaborations between pharmaceutical companies, academic institutions, and CROs can enhance research capabilities and accelerate the development of new therapies. Additionally, the expanding use of artificial intelligence and machine learning in clinical trials can improve data analysis and trial design, leading to more efficient and effective studies.
Challenges
Despite the promising opportunities, the neurology clinical trials market faces several challenges. Recruiting and retaining patients for clinical trials can be difficult, particularly for rare neurological disorders. The complexity of neurological diseases and the variability in disease progression pose challenges in designing and conducting trials. Regulatory hurdles and the need for stringent safety and efficacy assessments can delay the approval of new treatments. Additionally, the high costs associated with clinical trials and the competition for funding can be significant barriers for smaller companies and academic researchers. Addressing these challenges requires coordinated efforts from stakeholders across the healthcare ecosystem.
Read Also: Menstrual Health Apps Market Size to Grow USD 9.04 Bn by 2033
Recent Developments
- In August 2023, the phase II RECOVER-NEURO clinical trial study to evaluate the combination of REMOTE-transcranial direct current stimulation (tDCS) and a brain training program for long covid was launched by Soterix Medical.
- In March 2024, an innovative and new pTau217 blood test, ALZpath Dx, was launched by a specialized clinical laboratory, Neurocode USA, Inc., that offers world-class testing solutions for neurological disorders. This new test may be used in the monitoring, screening, and diagnosis of Alzheimer’s disease. In the US, Neurocode is the first laboratory to make this test as LDT (laboratory-developed test) for clinical trials, clinical diagnostics use, and other research causes.
Neurology Clinical Trials Market Companies
- IQVIA
- Biogen
- Aurora Healthcare
- GlaxoSmithKline Plc.
- Icon Plc.
- Syneous Health
- Charles River Laboratories
- Med pace
- Covance
- Novartis AG
- Sanofi
- Merck & Co., Inc.
- AbbVie Inc.
- Teva Pharmaceutical Industries Ltd.
- Annovis Bio
- Athira Pharma, Inc.
- Zydus Group
- Eli Lilly and Company
- Eisai Co., Ltd.
- AstraZeneca
- Supernus Pharmaceuticals, Inc. (Adamas Pharmaceuticals)
Segments Covered in the Report
By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By Study Design
- Interventional
- Observational
- Expanded Access
By Indication
- Epilepsy
- Parkinson’s Disease (PD)
- Huntington’s Disease
- Stroke
- Traumatic Brain Injury (TBI)
- Amyotrophic Lateral Sclerosis (ALS)
- Muscle Regeneration
- Others
By Study Design
- Epilepsy
- Interventional
- Observational
- Expanded Access
- Parkinson’s Disease (PD)
- Interventional
- Observational
- Expanded Access
- Huntington’s Disease
- Interventional
- Observational
- Expanded Access
- Stroke
- Interventional
- Observational
- Expanded Access
- Traumatic Brain Injury (TBI)
- Interventional
- Observational
- Expanded Access
- Amyotrophic Lateral Sclerosis (ALS)
- Interventional
- Observational
- Expanded Access
- Muscle Regeneration
- Interventional
- Observational
- Expanded Access
- Others
- Interventional
- Observational
- Expanded Access
by Phase
- Epilepsy
- Phase I
- Phase II
- Phase III
- Phase IV
- Parkinson’s Disease (PD)
- Phase I
- Phase II
- Phase III
- Phase IV
- Huntington’s Disease
- Phase I
- Phase II
- Phase III
- Phase IV
- Stroke
- Phase I
- Phase II
- Phase III
- Phase IV
- Traumatic Brain Injury (TBI)
- Phase I
- Phase II
- Phase III
- Phase IV
- Amyotrophic Lateral Sclerosis (ALS)
- Phase I
- Phase II
- Phase III
- Phase IV
- Muscle Regeneration
- Phase I
- Phase II
- Phase III
- Phase IV
- Others
- Phase I
- Phase II
- Phase III
- Phase IV
By Geography
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East & Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
Blog: https://www.dailytechbulletin.com/
Blog: https://www.autoindustrybulletin.com/
- Intelligent Traffic Management System Market Size, Trends, Report 2034 - July 17, 2024
- Healthcare Finance Solutions Market Size to Grow USD 293.12 Bn by 2034 - July 17, 2024
- Epoxy Composites Market Size to Grow USD 84.99 Bn by 2034 - July 17, 2024
