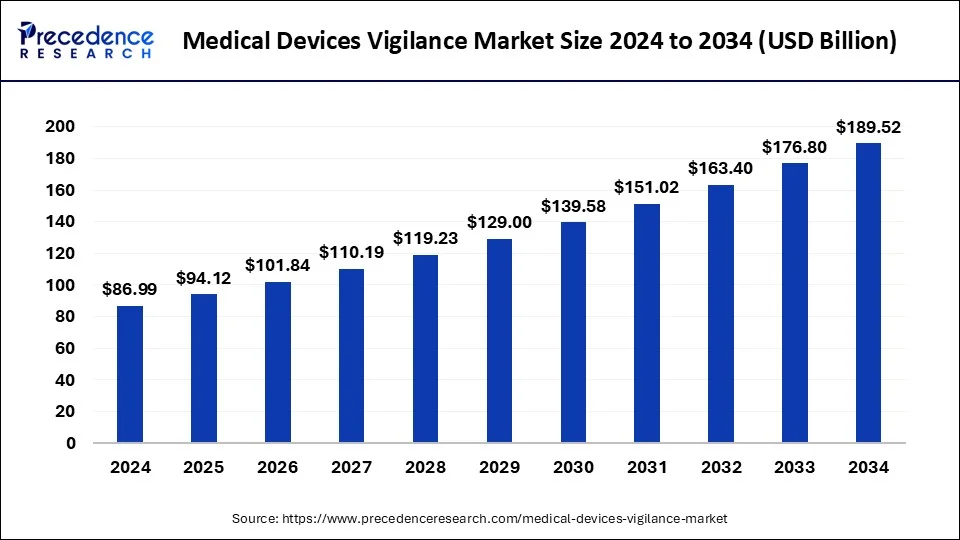
The global medical devices vigilance market size was estimated at USD 80.40 billion in 2023, growing at a CAGR of 8.19% from 2024 to 2033.
The medical devices vigilance market encompasses the processes and systems involved in monitoring and evaluating the safety and performance of medical devices after they have been placed on the market. This includes the collection, analysis, and dissemination of information related to adverse events, malfunctions, and potential risks associated with medical devices. The market aims to ensure the ongoing safety and effectiveness of these devices for patients and healthcare providers.
Key Points
- The North America medical devices vigilance market size reached USD 24.12 billion in 2023 and is expected to attain around USD 53.04 billion by 2033.
- North America was estimated to hold a substantial market share of 34% in 2023.
- Asia Pacific is projected to witness rapid growth in the global market.
- By delivery mode, the on-demand segment accounted for the largest share of 81% in 2023.
- By delivery mode, the on-premises segment is expected to have steady growth over the forecast period.
- By application, the diagnostics segment held a substantial market share of 36% in 2023.
- By application, the research segment is expected to show lucrative growth over the forecast period.
- By end use, the clinical research organization segment held the highest market share of 42% in 2023.
- By end use, the business process outsourcing firms segment is expected to grow rapidly in the foreseeable period.
Growth Factors:
The medical devices vigilance market is experiencing growth due to several factors. Increased regulatory requirements for post-market surveillance and reporting of adverse events have driven the need for robust vigilance systems. Additionally, the growing adoption of advanced medical technologies and the rising number of medical device-related incidents have further contributed to the market’s expansion. Technological advancements in data collection and analysis are also facilitating better vigilance practices.
Get a Sample: https://www.precedenceresearch.com/sample/4120
Region Insights: The market’s growth varies across different regions. In North America, strict regulatory frameworks and high adoption of advanced medical devices drive the vigilance market. Europe also exhibits significant growth due to stringent safety standards and a well-established regulatory system. In the Asia-Pacific region, rising healthcare infrastructure and increasing awareness about patient safety contribute to market growth. Emerging economies in Latin America and Africa are gradually catching up with global standards.
Medical Devices Vigilance Market Scope
| Report Coverage | Details |
| Growth Rate from 2024 to 2033 | CAGR of 8.19% |
| Global Market Size in 2023 | USD 80.40 Billion |
| Global Market Size in 2024 | USD 86.99 Billion |
| Global Market Size by 2033 | USD 176.80 Billion |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | By Delivery Mode, By Application, and By End-user |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Medical Devices Vigilance Market Dynamics
Drivers:
Key drivers of the medical devices vigilance market include:
- Stringent regulatory requirements for post-market surveillance.
- Increasing demand for safe and effective medical devices.
- Advancements in technology for data collection, analysis, and reporting.
- Growing adoption of connected medical devices, which necessitate continuous monitoring.
Opportunities: The market presents several opportunities for growth and development:
- Development of advanced vigilance software and platforms for better data management.
- Collaboration between regulatory bodies and industry players to streamline reporting processes.
- Integration of artificial intelligence and machine learning to enhance predictive analytics and risk assessment.
- Expansion into emerging markets with growing healthcare infrastructure.
Challenges: Despite its growth, the medical devices vigilance market faces certain challenges:
- Managing large volumes of data from various sources and ensuring its accuracy and relevance.
- Adapting to varying regulatory requirements across different regions.
- Balancing the cost of implementing advanced vigilance systems with the benefits of improved patient safety.
- Addressing concerns related to patient privacy and data security.
Read Also: Tire Pyrolysis Oil Market Size to Attain USD 567.90 Mn by 2033
Medical Devices Vigilance Market Recent Developments
- In June 2022, Italy instituted substantial changes in national regulations on medical device vigilance in accordance with the procedures by European Regulations. 2017/475 for medical devices and 2017/476 for in vitro diagnostics.
Medical Devices Vigilance Market Companies
- ZEINCRO
- AssurX Inc.
- Sparta System
- Oracle Corporation
- Xybion Corporation
- Sarjen Systems Pvt. Ltd.
- MDI Consultants, Inc.
- AB-Cube
- Laerdal Medical.
- Omnify Software, Inc.
Segments Covered in the Report
By Delivery Mode
- On-demand
- On-premise
By Application
- Diagnostics
- Therapeutics
- Surgical
- Research
By End-user
- Clinical Research Organizations (CROs)
- Business Process Outsourcing (BPO)
- Original Equipment Manufacturers (OEM)
- Other End-users
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
Blog: https://www.dailytechbulletin.com/
Blog: https://www.autoindustrybulletin.com/
- Quantum Dot Materials and Technologies Market to Surpass USD 46.4 Billion by 2034, Expanding at a CAGR of 22.64% - September 9, 2025
- Infrastructure For Business Analytics Market Size to Surpass USD 166.57 Bn by 2034, Growing at 9.05% CAGR - September 8, 2025
- Intelligent Traffic Management System Market Size, Trends, Report 2034 - July 17, 2024
