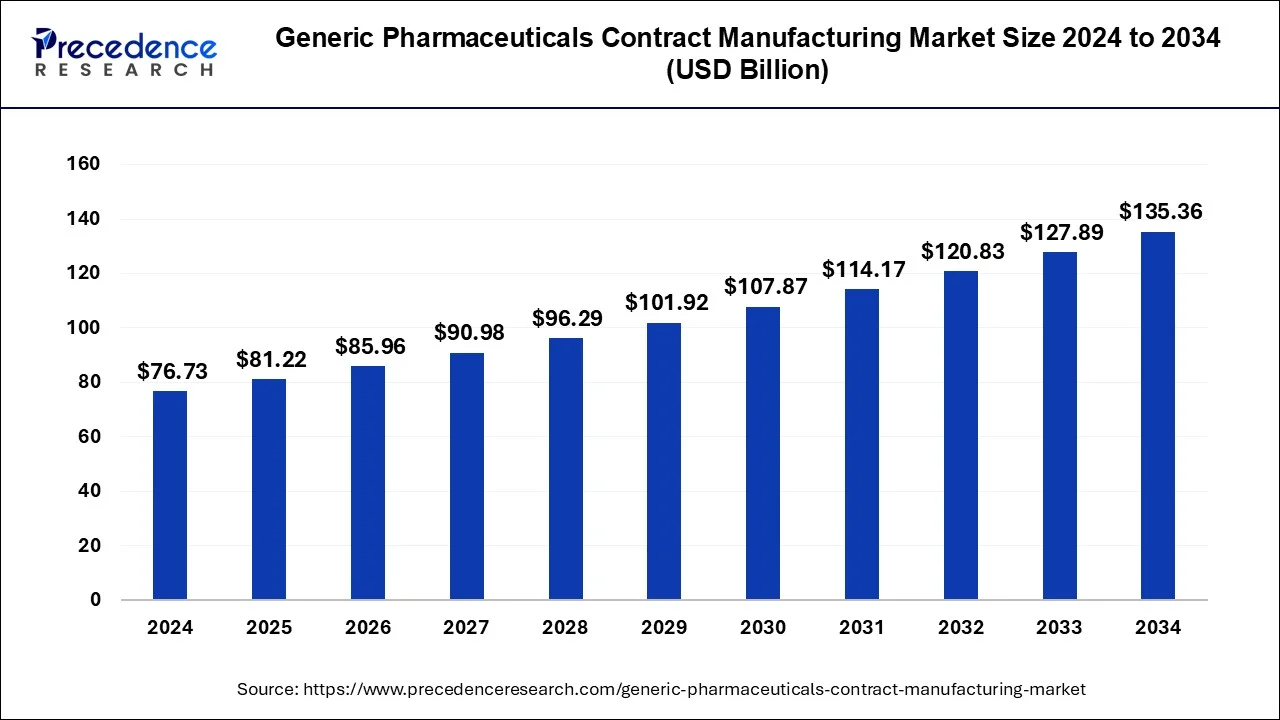
The global generic pharmaceuticals contract manufacturing market size accounted for USD 72.50 billion in 2023 and is predicted to attain around USD 135.36 billion by 2034, expanding at a CAGR of 5.84% from 2024 to 2034.
Generic Pharmaceuticals Contract Manufacturing Market Key Points
- North America is estimated to be the fastest-growing during the forecast period of 2024-2033.
- By drug type, the branded generics segment has contributed more than 63% of revenue share in 2023.
- By drug type, the unbranded generics segment is significantly growing during the forecast period.
- By product, the API product segment has held a major revenue share of 58% in 2023.
- By route of administration, the oral segment has captured the largest revenue share of 62% in 2023.
- By route of administration, the parenteral segment is anticipated to be the fastest-growing during the forecast period.
- By application, the oncology segment has generated the biggest revenue share of 23% in 2023.
- By application, the immunology segment is expected to be the fastest-growing during the forecast period.
The Generic Pharmaceuticals Contract Manufacturing Market has seen significant growth over the past decade, driven by the increasing demand for cost-effective healthcare solutions. Generic pharmaceuticals are bioequivalent to their branded counterparts, offering the same therapeutic benefits but at a lower cost. Contract manufacturing organizations (CMOs) play a crucial role in this market by providing manufacturing services to pharmaceutical companies that lack the capacity or capability to produce these drugs in-house. This market is characterized by its competitive nature, with numerous players vying for market share through quality manufacturing processes, regulatory compliance, and cost efficiency.
Regional Insights
North America: North America, particularly the United States, dominates the generic pharmaceuticals contract manufacturing market. The region’s robust healthcare infrastructure, coupled with a high prevalence of chronic diseases, drives the demand for generic drugs. Additionally, the presence of key market players and favorable regulatory policies by agencies such as the FDA contribute to market growth. The U.S. has a well-established pharmaceutical industry, with numerous companies outsourcing their manufacturing processes to CMOs to reduce costs and focus on core competencies such as R&D and marketing.
Europe: Europe is another significant market for generic pharmaceuticals contract manufacturing. Countries like Germany, the UK, and France lead the market due to their strong healthcare systems and high demand for affordable medications. The European Medicines Agency (EMA) ensures stringent regulatory standards, encouraging pharmaceutical companies to outsource manufacturing to specialized CMOs that can meet these standards. The rising healthcare costs and an aging population in Europe further fuel the demand for generic drugs, making it a lucrative market for contract manufacturers.
Asia-Pacific: The Asia-Pacific region is witnessing rapid growth in the generic pharmaceuticals contract manufacturing market. India and China are the major contributors due to their large pool of skilled labor, cost-effective manufacturing processes, and favorable government policies. India, known as the “pharmacy of the world,” has a robust generic pharmaceutical industry with many CMOs offering high-quality manufacturing services at competitive prices. The growing middle-class population and increasing healthcare awareness in the region also drive market growth.
Latin America and Middle East & Africa: These regions are emerging markets for generic pharmaceuticals contract manufacturing. Countries like Brazil and South Africa are experiencing increased demand for generic drugs due to economic constraints and the need for affordable healthcare solutions. The market in these regions is expected to grow as governments focus on improving healthcare infrastructure and access to essential medicines.
Generic Pharmaceuticals Contract Manufacturing Market Trends
Increased Outsourcing: One of the major trends in the market is the increased outsourcing of manufacturing processes by pharmaceutical companies. This trend is driven by the need to reduce operational costs, improve efficiency, and focus on core competencies such as research and development. CMOs offer specialized manufacturing capabilities and expertise, allowing pharmaceutical companies to bring products to market faster and more cost-effectively.
Technological Advancements: Technological advancements in manufacturing processes are transforming the generic pharmaceuticals contract manufacturing market. Innovations such as continuous manufacturing, automation, and advanced analytics are enhancing production efficiency, quality control, and scalability. These technologies enable CMOs to meet the stringent regulatory requirements and deliver high-quality generic drugs.
Regulatory Harmonization: The harmonization of regulatory standards across different regions is another significant trend. Regulatory bodies are working towards creating standardized guidelines for generic drug manufacturing, which simplifies the approval process and ensures consistency in product quality. This trend benefits CMOs by reducing the complexity and time required for regulatory compliance.
Focus on Quality and Compliance: Quality and regulatory compliance are critical factors in the generic pharmaceuticals contract manufacturing market. CMOs are increasingly focusing on implementing robust quality management systems and adhering to Good Manufacturing Practices (GMP) to meet regulatory requirements. This focus on quality ensures the production of safe and effective generic drugs, enhancing the reputation and credibility of CMOs.
Generic Pharmaceuticals Contract Manufacturing Market Scope
| Report Coverage | Details |
| Market Size by 2034 | USD 135.36 Billion |
| Market Size in 2023 | USD 72.50 Billion |
| Market Size in 2024 | USD 76.73 Billion |
| Market Growth Rate from 2024 to 2034 | CAGR of 5.84% |
| Largest Market | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2024 to 2034 |
| Segments Covered | Drug, Product, Route of Administration, Application, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Generic Pharmaceuticals Contract Manufacturing Market Dynamics
Drivers
Cost-Effectiveness: The primary driver of the generic pharmaceuticals contract manufacturing market is the cost-effectiveness of generic drugs compared to branded medications. As healthcare costs continue to rise, there is a growing demand for affordable treatment options. Generic drugs offer significant cost savings, making them an attractive choice for both healthcare providers and patients.
Patent Expirations: The expiration of patents for many blockbuster drugs creates opportunities for generic drug manufacturers. When a patent expires, other companies can produce and market generic versions of the drug, leading to increased competition and lower prices. CMOs play a crucial role in this process by providing the necessary manufacturing capabilities to produce generic versions of these drugs.
Increasing Prevalence of Chronic Diseases: The rising prevalence of chronic diseases such as diabetes, cardiovascular diseases, and cancer drives the demand for generic pharmaceuticals. These conditions require long-term treatment, and generic drugs provide cost-effective solutions for managing these diseases. The growing aging population also contributes to the increased demand for chronic disease treatments.
Government Initiatives and Healthcare Reforms: Governments worldwide are implementing initiatives and healthcare reforms to promote the use of generic drugs and reduce healthcare costs. These initiatives include policies that encourage the prescription of generic medications, streamline regulatory processes, and provide incentives for generic drug manufacturers. Such measures create a favorable environment for the growth of the generic pharmaceuticals contract manufacturing market.
Opportunities
Expansion in Emerging Markets: Emerging markets such as Asia-Pacific, Latin America, and Africa offer significant growth opportunities for the generic pharmaceuticals contract manufacturing market. These regions have a large population, increasing healthcare expenditure, and a growing demand for affordable medicines. CMOs can capitalize on these opportunities by establishing manufacturing facilities and partnerships in these regions.
Biosimilars Market: The biosimilars market presents a lucrative opportunity for generic pharmaceuticals contract manufacturers. Biosimilars are biologic drugs that are highly similar to already approved biologic drugs. As patents for biologic drugs expire, there is a growing demand for cost-effective biosimilars. CMOs with expertise in biologics manufacturing can tap into this market by offering biosimilar production services.
Partnerships and Collaborations: Collaborations between pharmaceutical companies and CMOs are becoming increasingly common in the generic pharmaceuticals contract manufacturing market. These partnerships enable pharmaceutical companies to leverage the manufacturing capabilities and expertise of CMOs while focusing on core activities such as research and development. CMOs can benefit from long-term contracts and a steady stream of business, driving mutual growth.
Technological Innovation: Continued investment in technological innovation presents significant opportunities for CMOs. Advanced manufacturing technologies, such as 3D printing, nanotechnology, and personalized medicine, are revolutionizing the pharmaceutical industry. CMOs that embrace these technologies can offer differentiated manufacturing services, attract new clients, and gain a competitive edge in the market.
Challenges
Regulatory Complexity: The generic pharmaceuticals contract manufacturing market is highly regulated, with stringent requirements imposed by regulatory bodies such as the FDA, EMA, and others. Navigating the complex regulatory landscape and ensuring compliance with varying regulations across different regions can be challenging for CMOs. Any lapses in regulatory compliance can lead to delays, product recalls, and reputational damage.
Quality Control and Assurance: Maintaining consistent quality and adhering to stringent quality control standards is a significant challenge for CMOs. Any deviation from quality standards can result in substandard products, regulatory penalties, and loss of client trust. CMOs must invest in robust quality management systems, employee training, and continuous monitoring to ensure high-quality manufacturing processes.
Supply Chain Disruptions: The global supply chain for pharmaceutical manufacturing is complex and susceptible to disruptions. Factors such as raw material shortages, transportation issues, and geopolitical tensions can impact the supply chain and lead to delays in production. CMOs need to have contingency plans in place and establish resilient supply chains to mitigate these risks.
Competition and Pricing Pressure: The generic pharmaceuticals contract manufacturing market is highly competitive, with numerous players vying for market share. Intense competition can lead to pricing pressure, affecting the profit margins of CMOs. To remain competitive, CMOs need to continuously improve operational efficiency, optimize costs, and offer value-added services to differentiate themselves from competitors.
Read Also: Facial Recognition Market Size to Attain USD 32.53 Bn by 2034
Generic Pharmaceuticals Contract Manufacturing Market Companies
- Metrics Contract Services
- Curia Global, Inc.
- Pfizer Centre One
- Syngene International Ltd.
- Acme Generics Pvt Ltd.
- Catalent, Inc.
- Alcami Corp., Inc.
- Cambrex Corp.
- Aurobindo Pharma
- Siegfried Holding AG
- Recipharm AB
- Jubilant Generics Ltd.
- Metrics Contract Services
Recent Developments
- In July 2023, Breyna Inhalation Aerosol, the first generic version of AstraZeneca’s Symbicort with an ANDA (abbreviated new drug application), was approved by the U.S. FDA (Food & Drug Administration) and launched by a global health company Viatris Inc. and Kindeva Drug Delivery L.P. for people with chronic obstructive pulmonary disease and asthma.
- In November 2023, an antibiotic manufacturing facility in Kundl, Austria was opened by a generic pharmaceuticals company based in Switzerland, Sandoz Group AG. In Europe, to strengthen the future of antibiotics manufacturing Sandoz invested $160.4m.
- In February 2024, in the United States, 5-6 new products in each quarter were planned to be launched by Alembic Pharmaceuticals, a Vadodara-based generics drugmaker.
Segments Covered in the Report
By Drug
- Branded Generics
- Unbranded Generics
By Product
- API
- Drug Product
By Route of Administration
- Oral
- Parenteral
- Topical
- Others
By Application
- Oncology
- Immunology
- Antidiabetic
- Neurology
- Anticoagulants
- Cardiovascular
- Respiratory
- Pain
- HIV antivirals
- Others
By Geography
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East & Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
Blog: https://www.dailytechbulletin.com/
Blog: https://www.autoindustrybulletin.com/
- Intelligent Traffic Management System Market Size, Trends, Report 2034 - July 17, 2024
- Healthcare Finance Solutions Market Size to Grow USD 293.12 Bn by 2034 - July 17, 2024
- Epoxy Composites Market Size to Grow USD 84.99 Bn by 2034 - July 17, 2024
