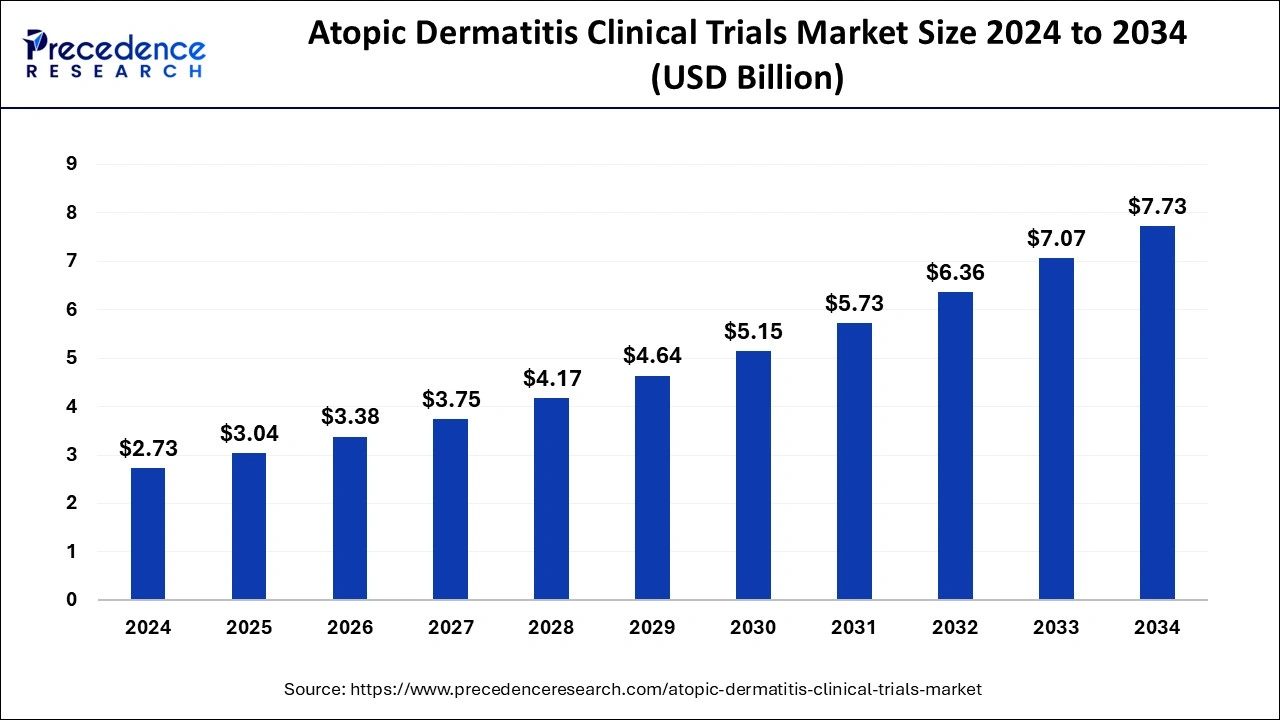
The global atopic dermatitis clinical trials market size is anticipated to be worth around USD 7.07 billion by 2033, expanding at a CAGR of 11.14% from 2024 to 2033.
Key Points
- North America has contributed more than 37% of market share in 2023.
- Asia-Pacific is estimated to expand the fastest CAGR between 2024 and 2033.
- By molecule type, the large molecules segment has held the largest market share of 54% in 2023.
- By molecule type, the small molecules segment is anticipated to grow at a remarkable CAGR of 12.5% between 2024 and 2033.
- By study design, the interventional trials segment has generated over 72% of market share in 2023.
- By study design, the observational trials segment is expected to expand at the fastest CAGR over the projected period.
- By phase, the phase II segment has accounted more than over 48% of market share in 2023.
- By phase, the phase III segment is expected to expand at the fastest CAGR over the projected period.
Atopic dermatitis, also known as eczema, is a chronic inflammatory skin condition characterized by itching, redness, and the development of rash-like patches on the skin. It is a common ailment affecting millions of individuals worldwide, with both children and adults susceptible to its symptoms. The burden of atopic dermatitis on patients’ quality of life, coupled with the absence of a definitive cure, has spurred extensive research and clinical trials aimed at developing more effective treatments and management strategies. The Atopic Dermatitis Clinical Trials Market encompasses the landscape of research endeavors dedicated to understanding the underlying mechanisms of the disease and evaluating novel therapeutic interventions.
Get a Sample: https://www.precedenceresearch.com/sample/4021
Growth Factors:
The Atopic Dermatitis Clinical Trials Market is experiencing significant growth driven by several factors. Firstly, the increasing prevalence of atopic dermatitis globally has heightened the demand for innovative treatment options. As awareness about the condition grows and diagnosis rates improve, there is a parallel surge in the number of patients seeking participation in clinical trials. Additionally, advancements in medical research techniques and technologies have facilitated the discovery of potential therapeutic targets, accelerating the pace of clinical trial development. Moreover, collaborations between pharmaceutical companies, academic institutions, and research organizations have fostered a collaborative environment conducive to the progression of clinical research in atopic dermatitis.
Region Insights:
Clinical trials for atopic dermatitis are conducted across various regions worldwide, reflecting the global nature of the disease burden and research efforts. North America, particularly the United States, occupies a prominent position in the Atopic Dermatitis Clinical Trials Market, owing to its robust healthcare infrastructure, large patient population, and extensive research funding. Europe also plays a significant role in clinical trial activities, with countries such as Germany, the United Kingdom, and France emerging as key hubs for dermatological research. In the Asia-Pacific region, rapid economic development, increasing healthcare expenditure, and rising prevalence of atopic dermatitis are driving the expansion of clinical trial activities, particularly in countries like Japan, China, and South Korea.
Atopic Dermatitis Clinical Trials Market Scope
| Report Coverage | Details |
| Growth Rate from 2024 to 2033 | CAGR of 11.14% |
| Global Market Size in 2023 | USD 2.46 Billion |
| Global Market Size by 2033 | USD 7.07 Billion |
| U.S. Market Size in 2023 | USD 640 Million |
| U.S. Market Size by 2033 | USD 1,830 Million |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | By Molecule Type, By Study Design, and By Phase |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Atopic Dermatitis Clinical Trials Market Market Dynamics
Drivers:
Several drivers propel the growth of the Atopic Dermatitis Clinical Trials Market. One of the primary drivers is the unmet medical need associated with atopic dermatitis. Despite the availability of treatments, many patients continue to experience inadequate symptom relief or adverse effects from existing therapies. This underscores the urgency for novel therapeutic options, spurring investment in clinical research. Furthermore, regulatory agencies’ emphasis on expediting the development and approval of innovative treatments for dermatological conditions has incentivized pharmaceutical companies to invest in atopic dermatitis clinical trials. Additionally, the growing acceptance of personalized medicine approaches and targeted therapies has fueled interest in identifying biomarkers and developing tailored treatment strategies through clinical research.
Opportunities:
The Atopic Dermatitis Clinical Trials Market presents numerous opportunities for stakeholders across the healthcare spectrum. For pharmaceutical companies, investing in atopic dermatitis clinical trials offers the potential for market differentiation and revenue generation through the introduction of novel therapeutics. Academic institutions and research organizations have the opportunity to contribute to scientific advancements in the field while enhancing their reputation as leaders in dermatological research. Healthcare providers can benefit from participating in clinical trials by gaining access to cutting-edge treatments and contributing to improved patient outcomes. Furthermore, patients themselves stand to benefit from participation in clinical trials by gaining early access to potentially transformative therapies and contributing to the advancement of medical knowledge.
Challenges:
Despite the promising outlook, the Atopic Dermatitis Clinical Trials Market is not without its challenges. One significant challenge is the complexity of atopic dermatitis as a disease, which encompasses diverse clinical phenotypes and underlying pathophysiological mechanisms. This heterogeneity poses challenges for clinical trial design, patient selection criteria, and treatment response assessment. Moreover, recruiting and retaining participants for clinical trials can be challenging due to factors such as patient reluctance, geographic dispersion, and stringent eligibility criteria. Additionally, the regulatory landscape governing clinical research poses challenges in terms of navigating complex approval processes, ensuring compliance with regulatory requirements, and addressing safety concerns effectively.
Read Also: Urinalysis Market Size to Cross USD 3.65 Billion by 2033
Recent Developments
- In October 2023, LEO Pharma disclosed the favorable outcome of the DELTA 3 trial. This Phase 3 extension trial assessed delgocitinib cream, an investigational topical pan-Janus kinase (JAK) inhibitor, for potential treatment in adults with moderate to severe chronic hand eczema (CHE). This positive result bolstered the company’s pipeline value and is expected to facilitate the commercialization of the product.
- In May 2023, Dermavant Sciences, Inc. unveiled encouraging results from ADORING 1, Phase 3 studies investigating the efficacy and safety of topical VTAMA (tapinarof) cream 1% in adults and pediatric subjects as young as 2 years old with moderate to severe atopic dermatitis. These findings provided the company with a route to commercial success, expansion in the market, competitive differentiation, and increased investor support.
Atopic Dermatitis Clinical Trials Market Companies
- Pfizer Inc.
- Sanofi SA
- Regeneron Pharmaceuticals, Inc.
- AbbVie Inc.
- Novartis International AG
- Eli Lilly and Company
- GlaxoSmithKline plc
- Johnson & Johnson
- Leo Pharma A/S
- Dermira, Inc. (acquired by Eli Lilly and Company)
- Galderma S.A.
- AnaptysBio, Inc.
- Dermavant Sciences, Inc.
- LEO Pharma A/S
- Amgen Inc.
Segments Covered in the Report
By Molecule Type
- Small Molecules
- Large Molecules
By Study Design
- Interventional
- Observational
By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
Blog: https://www.dailytechbulletin.com/
Blog: https://www.autoindustrybulletin.com/
- Intelligent Traffic Management System Market Size, Trends, Report 2034 - July 17, 2024
- Healthcare Finance Solutions Market Size to Grow USD 293.12 Bn by 2034 - July 17, 2024
- Epoxy Composites Market Size to Grow USD 84.99 Bn by 2034 - July 17, 2024
