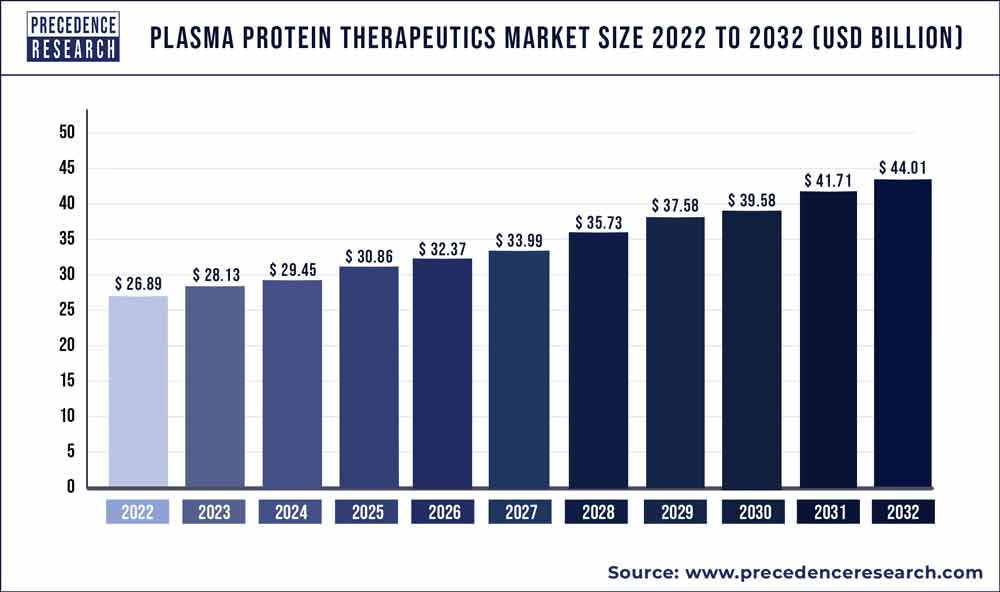
The global plasma protein therapeutics market size is projected to be worth around USD 44.01 billion by 2032 from USD 28.13 billion in 2023, rising at a CAGR of 5.10% from 2023 to 2032.
Key Takeaways
- North America contributed more than 47% of revenue share in 2022.
- Asia-Pacific is estimated to expand the fastest CAGR between 2023 and 2032.
- By product type, the immunoglobulin segment has held the largest market share of 35% in 2022.
- By product type, the albumin segment is anticipated to grow at a remarkable CAGR of 6.5% between 2023 and 2032.
- By application, the hemophilia segment generated over 32% of revenue share in 2022.
- By application, the primary immunodeficiency disorder segment is expected to expand at the fastest CAGR over the projected period.
- By end user, the hospitals segment generated over 70% of revenue share in 2022.
- By end user, the other segment is expected to expand at the fastest CAGR over the projected period.
The Plasma Protein Therapeutics Market revolves around the development and commercialization of therapeutic products derived from human plasma. These products play a crucial role in treating various rare and chronic medical conditions. As the demand for advanced healthcare solutions continues to rise globally, the market for plasma protein therapeutics has gained significant traction.
Growth Factors:
Several key factors contribute to the growth of the Plasma Protein Therapeutics Market. First and foremost is the increasing prevalence of chronic diseases, creating a growing patient pool in need of effective treatments. Advances in biotechnology and protein purification techniques have also played a pivotal role, enhancing the development and production of high-quality plasma-derived therapeutics. Additionally, the rising awareness among healthcare professionals and patients about the benefits of these therapies contributes to sustained market growth.
Get a Sample: https://www.precedenceresearch.com/sample/3630
Plasma Protein Therapeutics Market Scope
| Report Coverage | Details |
| Growth Rate from 2023 to 2032 | CAGR of 5.10% |
| Market Size in 2023 | USD 28.13 Billion |
| Market Size by 2032 | USD 44.01 Billion |
| Largest Market | North America |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Segments Covered | By Product Type, By Application, and By End-user |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Opportunities:
The Plasma Protein Therapeutics Market presents promising opportunities on multiple fronts. The expansion of therapeutic applications beyond traditional uses, such as immunodeficiency disorders, into areas like neurology and hematology, opens up new avenues for growth. Furthermore, the untapped potential in emerging markets, coupled with increased research and development efforts, creates opportunities for market players to diversify their product portfolios and reach a broader patient base.
Challenges:
Despite its growth, the market faces challenges that require careful navigation. Regulatory complexities and stringent safety standards associated with plasma-derived products can pose hurdles for market entry and product approval. Additionally, the potential risk of transmission of infectious agents through plasma-derived therapies remains a concern, necessitating continuous advancements in screening and purification processes to ensure product safety. Moreover, the high costs associated with the development and manufacturing of these therapies may limit accessibility for certain patient populations. Addressing these challenges will be essential for sustained success in the Plasma Protein Therapeutics Market.
By Product Type:
Immunoglobulins (Ig): Immunoglobulins, or antibodies, constitute a significant product type within the Plasma Protein Therapeutics Market. Used in the treatment of immunodeficiency disorders, autoimmune diseases, and infections, the demand for immunoglobulins continues to rise with increasing awareness and prevalence of these conditions.
Clotting Factor Concentrates: Another crucial product type is clotting factor concentrates, essential for individuals with bleeding disorders like hemophilia. These concentrates derived from plasma play a vital role in managing and preventing bleeding episodes, contributing to the well-being of patients with clotting disorders.
Albumin: Albumin, a versatile plasma protein, serves various medical purposes. Used in fluid volume restoration, therapeutic plasma exchange, and as a drug carrier, albumin finds applications across a range of medical conditions. The increasing incidence of liver diseases and an aging population contribute to the growth of the albumin segment.
Protease Inhibitors and Alpha-1 Antitrypsin: Protease inhibitors, employed in treating conditions such as HIV/AIDS, and alpha-1 antitrypsin, used to manage genetic disorders affecting the lungs and liver, represent additional product types catering to specific medical needs.
By Application:
Immunodeficiency Disorders: Immunoglobulins play a crucial role in treating immunodeficiency disorders, where the immune system is compromised. The application of immunoglobulin therapies helps bolster the immune response and enhance the overall health of individuals with these conditions.
Bleeding Disorders: Clotting factor concentrates are specifically applied in the management of bleeding disorders like hemophilia. These concentrates assist in preventing and controlling bleeding episodes in patients, contributing to improved quality of life.
Liver Diseases and Therapeutic Plasma Exchange: Albumin finds application in addressing liver diseases and is used in therapeutic plasma exchange procedures. The versatile nature of albumin allows it to play a role in multiple medical applications, expanding its utility in diverse healthcare settings.
By End-user:
Hospitals and Clinics: Hospitals and clinics serve as major end-users of Plasma Protein Therapeutics, offering a range of treatments to patients. The accessibility and infrastructure of healthcare facilities contribute to the utilization of plasma protein therapies in these settings.
Homecare Settings: With advancements in technology and the development of self-administered therapies, some plasma protein treatments, especially for chronic conditions, are administered in homecare settings. This approach provides convenience and flexibility for patients.
Research and Academic Institutes: Research and academic institutes play a role in advancing the field of plasma protein therapeutics through ongoing research and development. These institutions contribute to the discovery of new therapies and the enhancement of existing ones, driving innovation in the market.
Read Also: Radar Sensors Market Size To Grow USD 39.37 Billion by 2032
Recent Developments
- In December 2022, Biotest AG revealed the initiation of a clinical phase II pilot study for treating chronic hepatitis B with hyperimmunoglobulins at the Hanover Medical School, enrolling the first of 20 patients.
- In June 2022, Biotest AG reported a successful interim analysis of the phase III AdFIrst trial, assessing Fibrinogen in patients with acquired fibrinogen deficiency.
- In May 2020, Biotest AG completed the clinical trial 984, treating patients with congenital fibrinogen deficiency using the fibrinogen concentrate (BT524) for acute bleeding or prophylactic treatment before surgery.
- In July 2022, Takeda announced the positive outcome of the ADVANCE-1 Phase 3 clinical trial for HYQVIA in the maintenance treatment of chronic inflammatory demyelinating polyradiculoneuropathy (CIDP).
- In November 2022, Biotest AG received approval from the Paul-Ehrlich-Institute for the new intravenous immunoglobulin Yimmugo (IgG Next Generation) in Germany.
- In January 2022, Bio Products Laboratory (BPL) obtained a license from the NMPA to market ALBUMINEX 25% product in China.
- In March 2022, Grifols announced the approval of XEMBIFY, its 20% subcutaneous immunoglobulin, by several European Union member state health authorities and the U.K., to treat primary and select secondary immunodeficiency.
- In April 2022, Grifols completed the acquisition of Tiancheng (Germany) Pharmaceutical Holdings AG, which holds a significant stake in Biotest AG.
- In September 2022, Grifols signed a long-term agreement with Canadian Blood Services to enhance Canada’s self-sufficiency in immunoglobulin medicines.
Plasma Protein Therapeutics Market Players
- CSL Limited
- Grifols S.A.
- Takeda Pharmaceutical Company Limited
- Octapharma AG
- Biotest AG
- Kedrion S.p.A.
- Shire (acquired by Takeda)
- Bio Products Laboratory (BPL)
- Kamada Ltd.
- China Biologic Products Holdings, Inc.
- Octapharma Plasma, Inc.
- Sanquin
- LFB S.A.
- ADMA Biologics, Inc.
- Bioverativ (acquired by Sanofi)
Segments Covered in the Report
By Product Type
- Immunoglobulin
- Albumin
- Plasma derived factor VIII
- Others
By Application
- Hemophilia
- Idiopathic thrombocytopenic purpura
- Primary immunodeficiency disorder
- Others
By End-user
- Hospitals
- Others
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
Blog: https://www.dailytechbulletin.com/
Blog: https://www.autoindustrybulletin.com/
- Photodynamic Therapy Market Size to Rake USD 8.42 Bn by 2033 - February 5, 2024
- Image Recognition Market Size to Attain USD 166.01 Bn by 2033 - February 5, 2024
- Hydrogen Storage Tanks and Transportation Market Report 2033 - February 5, 2024
